Abstract
Background: Two chimeric antigen receptor (CAR) T cell therapies, liso-cel and axi-cel, demonstrated superior efficacy over salvage chemotherapy and autologous transplant for chemotherapy sensitive disease as 2L therapy in transplant-intended pts with high-risk R/R LBCL, yet no head-to-head clinical trials have compared CAR T cell therapies. A previous MAIC report showed comparable efficacy and an improved safety profile of liso-cel vs axi-cel in 3L+ R/R LBCL (Maloney et al. J Hematol Oncol 2021). Here we conducted a MAIC of treatment effects in the 2L setting for liso-cel vs axi-cel in pts with R/R LBCL.
Methods: MAICs were used to estimate population-adjusted relative treatment effects associated with liso-cel for event-free survival (EFS), PFS, ORR, and CR rate (TRANSFORM; NCT03575351; N = 184) vs axi-cel (ZUMA-7; NCT03391466; N = 359), as well as safety (TRANSFORM, N = 183; ZUMA-7, N = 338). Pts from TRANSFORM were excluded from this analysis if they did not satisfy eligibility criteria specified in ZUMA-7 (ie, matching). Individual pt data (IPD) for pts who remained in the TRANSFORM data set were weighted using a method-of-moments propensity score model to match the marginal distribution (ie, mean, variance) of clinical factors among pts from ZUMA-7 (ie, adjustment). Baseline characteristics and outcome measures were revised to align with those defined in ZUMA-7. Efficacy comparisons were anchored through the common comparator, standard-of-care (SOC; with similar protocol-defined salvage chemotherapy regimens in both trials, followed by high-dose chemotherapy and autologous transplant in responders). Hazard ratios (HRs) were used to compare time to event outcomes (EFS, PFS), and odds ratios (OR) were used to compare binary outcomes (ORR, CR rate, safety). Selection and rank-ordering of the treatment effect modifiers were guided by analysis of the TRANSFORM IPD and by a panel of expert clinicians. Factors to match (ie, pts from TRANSFORM were removed) and adjust (ie, pts from TRANSFORM were re-weighted) for efficacy included: central nervous system involvement, absolute lymphocyte count at screening, age, sex, geographic region, secondary age-adjusted International Prognostic Index (sAAIPI) score, sum of the product of perpendicular diameters at baseline, R/R status to 1L therapy, double/triple-hit status, and disease histology. Safety comparisons were unanchored, owing to the absence of CAR T cell-associated toxicities in the SOC arms; factors for safety MAIC included: LVEF < 50% and bilirubin > 1.5 mg/dL at screening, age, and sAAIPI score. Notably, bridging chemotherapy was allowed in TRANSFORM but not in ZUMA-7; it was not practically possible to adjust for this factor given sample size constraints.
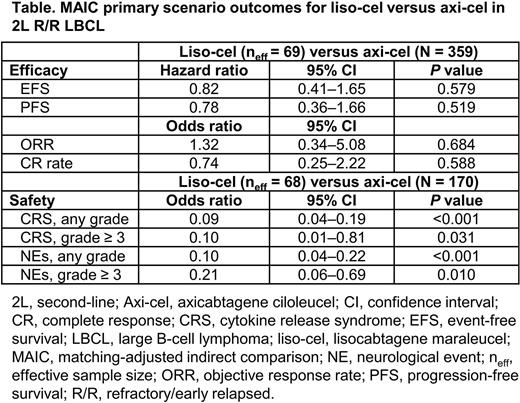
Results: Unmatched and unadjusted comparisons showed no differences in median (95% CI) EFS for liso-cel (10.1 mo [6.1‒not reached]) vs axi-cel (8.3 mo [4.5‒15.8]; HR, 0.94 [0.58‒1.52]). After matching with ZUMA-7 for pt eligibility, the TRANSFORM sample size was 157; matching and adjusting for the selected effect modifiers resulted in an effective sample size of 69 for the primary efficacy scenario comparisons (sample size unchanged for ZUMA-7). MAIC primary scenario efficacy results for these populations showed no differences (Table). Median (95% CI) EFS after matching and adjustment was 9.5 mo (5.95‒not reached) for liso-cel. Results were consistent for other efficacy parameters of PFS, ORR, and CR rate. Sensitivity scenario comparisons that included additional adjustment factors led to similar results. MAIC safety results demonstrated lower odds of key CAR T cell-associated AEs with liso-cel vs axi-cel: cytokine release syndrome (CRS) any grade (OR, 0.09 [95% CI: 0.04‒0.19]), CRS grade ≥ 3 (OR, 0.10 [0.01‒0.81]), neurological events (NE) any grade (OR, 0.10 [0.04‒0.22]), and NEs grade ≥ 3 (OR, 0.21 [0.06‒0.69]).
Conclusions: Findings from this comparative analysis of liso-cel and axi-cel for the 2L treatment of R/R LBCL show comparable efficacy of liso-cel and axi-cel, with more favorable safety outcomes for liso-cel where lower rates of all-grade and grade ≥ 3 CRS and NEs were observed. These results indicate liso-cel may have a better safety profile than axi-cel in 2L+ R/R LBCL and are consistent with those from the 3L+ MAIC analysis. This MAIC will be updated with the TRANSFORM primary analysis dataset when available.
Disclosures
Abramson:Bluebird bio: Consultancy; Kymera: Consultancy; BeiGene: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Abbvie: Consultancy; BMS: Consultancy, Research Funding; Incyte: Consultancy; Kite Pharma: Consultancy; Genmab: Consultancy; Ono Pharma: Consultancy; Mustang Bio: Consultancy; MorphoSys: Consultancy; Regeneron: Consultancy; Century: Consultancy; Astra-Zeneca: Consultancy; Lilly: Consultancy; Janssen: Consultancy; Seattle Genetics: Research Funding. Kamdar:TG Therapeutics: Consultancy, Research Funding; Beigene: Consultancy; SeaGen: Speakers Bureau; BMS: Consultancy; AstraZeneca: Consultancy; Impact Bio: Consultancy; Abbvie: Consultancy; Novartis: Research Funding; Celgene: Other: DMC; Genentech: Consultancy, Other: DMC, Research Funding; Adaptive Technologies: Consultancy; ADC Therapeutics: Consultancy. Liu:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Crotta:BMS: Current Employment, Current equity holder in publicly-traded company. Previtali:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Klijn:BMS: Current Employment; OPEN Health: Ended employment in the past 24 months. Wang:EVERSANA: Current Employment, Other: EVERSANA is a consultancy that is contracted to and receives funds from BMS and I am a paid employee of EVERSANA. ; BMS: Consultancy. Situ:BMS: Consultancy. Zhang:EVERSANA: Current Employment, Other: EVERSANA is a consultancy that is contracted to and receives funds from BMS and I am a paid employee of EVERSANA; BMS: Consultancy. Bonner:EVERSANA: Other: EVERSANA is a consultancy that is contracted to and receives funds from BMS and I am a paid employee of EVERSANA ; BMS: Consultancy. Lunning:EUSA: Consultancy; Acrotech: Consultancy; Astra-Zeneca: Consultancy; TG Therapeutics: Consultancy; CURIS: Research Funding; Seattle Genetics: Consultancy; Nurix Therapeutics: Consultancy; Genentech: Consultancy; Morphosys: Consultancy; Diiachi-Sankyo: Consultancy; Janssen: Consultancy; Kite, a Gilead Company: Consultancy; ADC Therapeutics: Consultancy; Genmab: Consultancy; Fate Therapeutics: Consultancy; AbbVie: Consultancy; Astellas: Consultancy; BMS: Consultancy, Research Funding; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal